- Online Patient Services
- Patient Booklet
- e - Medical Records
- Online Expert Opinion-NAVYA
- Donation to TMC
- Online Hospital Statistics
- Pensioner Portal
- Travel Facility
- Schedule of Charges
- FAQs
- International Patients Advisor
- TMC - Annual Report
- TMC -Performance Statistics
- TMH Facts: average per day
- Classification of Surgeries
- D.A.E
- Govt of India Sites :
- International Affiliations
- Pancreatic Cancer India
- Tobacco Act
- Observership Application
- Teleconsulting Services
- TMC
- Home
- About Us
- Cancer Information
- Patient Services
- Departments
- NCG
- Disease Management Group
- Education and Research
- Events
Paediatric Hemato Lymphoid
- Members
- Service
- Meetings/OPD
- Research
- News & Events
- Guidelines & Patient Information
- Contact Us
Convener : Dr. Chetan Dhamne (Pediatric Medical Oncology )
Secretary : Dr. Prashant Tembhare (Hematopathology)
Pediatric Medical Oncology
- Dr. Shripad D Banavali
- Dr. (Surg Cdr) Gaurav Narula
- Dr. Chetan Dhamne
- Dr. Nirmalya Roy Moulik
- Dr. Badira Cheriyankil Parambil
- Dr. Akanksha Chichra
- Dr. Venkata Ram Mohan Gollamudi
- Dr. Shyam Shrinivasan
Radiation Oncology
- Dr. Siddharth Laskar
- Dr. Sangeeta Kakoti
Surgical Oncology
- Dr. Sajid Qureshi
Hemato-Pathology
- Dr. Sumeet Gujral
- Dr. P. G. Subramanian
- Dr. PrashantTembhare
- Dr. Gaurav Chatterjee
- Dr. Shweta Rajpal
- Dr. Nikhil Patkar
- Dr. Sridhar Epari
Histopathology
- Dr. Tanuja Shet
- Dr. Sridhar Epari
Cytogenetics
- Dr. Dhanlaxmi Shetty
- Ms.Purvi Mohanty
Medical Oncology &Transplantation
- Dr. Navin Khattry
Microbiologist
- Dr. Sanjay Biswas
- Dr. Gaurav Salunke
Radiology & Nuclear Medicine
- Dr. Venkatesh Rangarajan
- Dr. Sneha Shah
Radiology
- Dr. Akshay Baheti
- Dr. Vasundhara Patil
Psychiatry and Clinical Psychology
- Dr. Joyita Deodhar
Clinical Pharmacology
- Dr. Vikram Gota
- Dr. Manjunath N
Research Scientist Hasan Lab
- Dr. Syed Hasan
Interventional Radiology
- Dr. Nitin Shetty
- Dr. Raghunath Nagvekar
Anesthesiology and Intensive Care
- Dr. Vijaya Patil
Key Cancer Benchmarks: Prospective epidemiological and disease data for all patients registered in the PHLG DMG is being captured according to specified proforma and entered on IPOD database (as specified in operational policy). This is being audited every 6 months initially and now every monthly to look at the trends in disease volumes, treatment compliance and completion, treatment delivery, mortality and morbidity rates and survival of each disease subcategory in PHLG. The details and trends for year 2016 are as below:
Volume Indicators:
Pediatric HLG sees more than 800 patients every year, which is the highest in the country and among the highest in the world. The cumulative patient visits every year exceeds 35,000 as majority of these require intense, curative and prolonged therapies with multiple outpatient and inpatient visits. A total of 887 new patients were registered and 35210 cases were seen during follow-up in 2016. There were 675 new cases of acute leukemias and 174 new cases of lymphomas which are among the highest seen by any Pediatric cancer unit in the world.
|
DISEASE |
Disease Burden In 2016 |
|||
|---|---|---|---|---|
|
|
General |
Private |
TOTAL |
(%) |
|
ALL |
461 |
86 |
547 |
61.7 |
|
AML |
113 |
15 |
128 |
14.4 |
|
CML |
13 |
1 |
14 |
1.6 |
|
NHL |
81 |
12 |
93 |
10.5 |
|
HL |
67 |
14 |
81 |
9.1 |
|
LCH |
6 |
3 |
9 |
1.0 |
|
JMML |
15 |
0 |
15 |
1.7 |
|
TOTAL |
756 |
131 |
887 |
100 |
Outcome Indicators:
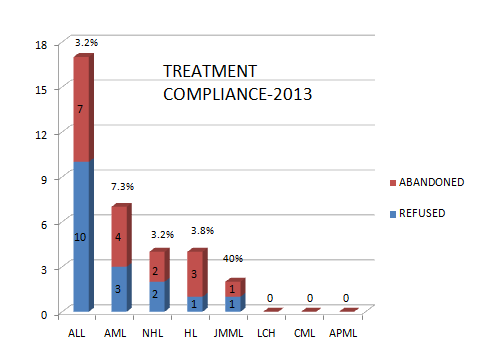
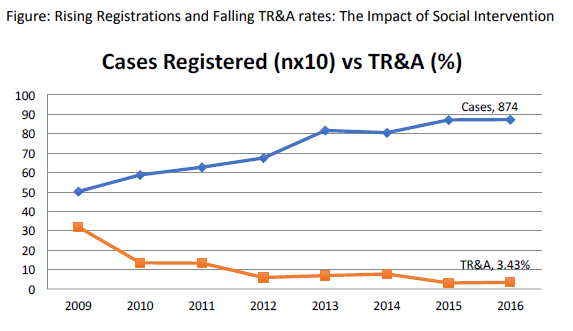
Treatment refusal and abandonment: The treatment refusal and abandonment (TRA) rates have been more than 20% in 2009. This has been due to multiple reasons including lack of money for therapy, lack of accommodation during long treatment, lack of free transport, lack of care providers for siblings at home, loss of job of parents with no money for subsistence, lack of transfusion support and ignorance regarding curability of cancer etc. In 2010, PHLG set up the social support group to provide holistic care of children and their family during treatment consisting of social workers, data managers for patient tracking, counsellors, psychologists, and multiple NGOs to ensure accommodation, transfusion support, education etc. This has dramatically decreased the abandonment rates from >20% in 2009 to 9.5% in 2010 and 5.17% in 2012. This has further reduced to 4% in 2014. The current TRA is primarily in diseases requiring expensive therapy with very poor outcome. This highly successful model of support won the international society of Pediatric Oncology (SIOP) award at London meeting in 2012. The overall induction mortality rates in leukemias have reduced from 8% in 2010 to consistently below 4.5% since 2013 by a process of triage, constant supervision & aggressive management of treatment related complications. Number of patients being treated as per standard TMH protocols has improved from 79% in 2010 to 89% by 2013, and above 90% since 2014.with reduction in the rates of TR&A and second opinions. The Pediatric cancer registry maintains details of epidemiology and outcomes of all Pediatric hematolymphoid cancers.
|
DISEASE |
TREATMENT COMPLIANCE IN 2016 |
|||
|---|---|---|---|---|
|
|
REFUSED |
ABANDONED |
TOTAL |
(%) |
|
ALL |
12 |
1 |
13 |
2.8 |
|
AML |
6 |
3 |
9 |
7.0 |
|
CML |
0 |
0 |
0 |
0.00 |
|
NHL |
0 |
1 |
1 |
1.1 |
|
HL |
2 |
0 |
2 |
2.5 |
|
LCH |
0 |
0 |
0 |
0.00 |
|
JMML |
1 |
0 |
1 |
6.7 |
|
MPD |
0 |
0 |
0 |
0.00 |
|
TOTAL |
21 |
5 |
26 |
0.03 |
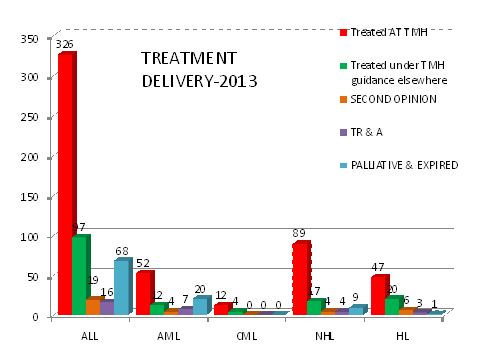
A)Treatment Delivery: As evident from table detailing the treatment compliance in 2013; in 2012,79% were being treated as per standard TMH protocols either at TMH (56%) or other hospitals under TMH guidance (23.2%). This number has improved to 85% in 2013 with reduction in the rates of TRA and second opinions. Approximately 4% & 5% patients died before starting therapy in 2012 & 2013 respectively largely due to advanced disease. Currently, 66% patients are being treated as per TMH protocols at TMH and 19% at other hospitals under TMH guidance. We have consistently exceeded 90% since 2013-14.
|
DISEASE |
TOTAL NO. |
2016 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Treated AT TMH |
Treated under TMH guidance elsewhere |
SECOND OPINION |
TR & A |
PALLIATIVE & EXPIRED |
||||||
|
|
|
(%) |
(%) |
(%) |
(%) |
(%) |
|||||
|
ALL |
547 |
451 |
82.44 |
36 |
6.58 |
47 |
8.6 |
13 |
2.3 |
60 |
13.3 |
|
AML |
128 |
102 |
79.68 |
7 |
5.4 |
14 |
10.93 |
9 |
7.0 |
34 |
33.33 |
|
CML |
14 |
13 |
100 |
0 |
0 |
0 |
0.00 |
0 |
0.0 |
1 |
7.69 |
|
NHL |
93 |
82 |
88.1 |
8 |
8.6 |
3 |
3.22 |
1 |
1.1 |
15 |
18.29 |
|
HL |
81 |
61 |
88.17 |
10 |
12.34 |
10 |
12.34 |
2 |
2.5 |
4 |
6.55 |
|
LCH |
9 |
7 |
77.77 |
1 |
11.11 |
1 |
11.11 |
0 |
0 |
1 |
14.28 |
|
JMML |
15 |
12 |
80 |
0 |
0.00 |
2 |
13.33 |
1 |
6.66 |
6 |
50 |
|
Total |
887 |
728 |
82.13 |
62 |
7 |
77 |
8.6 |
26 |
2.3 |
121 |
13.6 |
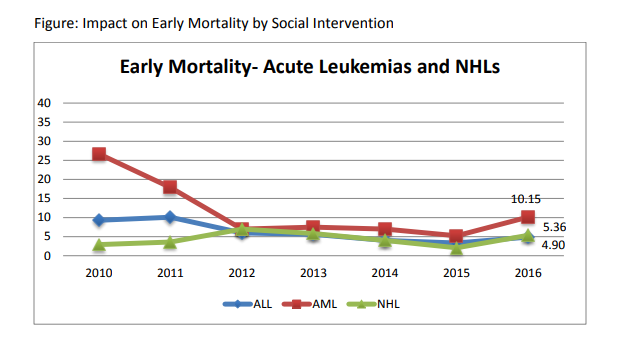
B)Mortality: In year 2010, the mortality rates were very high. The overall induction mortality rates were 8% with approximately 10% patients dying in ALL and 16% in AML during induction period. Most of these deaths have been toxic deaths due to infection. This has been reduced to 4.8% in 2013 with 5% & 11% induction deaths in ALL & AML respectively. Similarly, the post induction mortality which represents late events of toxic deaths as well as relapses has been reduced from 18% to 3.2%. We hope to achieve less than 5% induction mortality in ALL & AML and reduce our post induction mortality through reduction of relapse deaths by refining our protocols further in 2013-14. The disease free survival amongst BMT patients is 70% and transplant related mortality is 6%.
|
DISEASE |
TOTAL |
TOTAL |
Mortality in 2016 |
|||||
|---|---|---|---|---|---|---|---|---|
| BEFORE RX | WITHIN 45 DAY'S | AFTER 45 DAY'S | ||||||
| NO. | (%) | NO. | (%) | NO. | (%) | |||
| ALL | 547 | 52 | 13 | 2.3 | 27 | 4.9 | 25 | 4.57 |
| AML | 128 | 26 | 5 | 3.9 | 13 | 10.15 | 13 | 10.15 |
| CML | 13 | 1 | 1 | 7.69 | 1 | 7.69 | 0 | 0 |
| NHL | 93 | 10 | 3 | 3.22 | 5 | 5.36 | 5 | 5.37 |
| HL | 81 | 4 | 1 | 1.23 | 4 | 4.93 | 0 | 0 |
| LCH | 9 | 1 | 1 | 11.11 | 1 | 11.11 | 0 | 0 |
| JMML | 15 | 4 | 2 | 13.33 | 3 | 20 | 1 | 6.66 |
| TOTAL | 757 | 98 | 26 | 3.43 | 54 | 7.13 | 44 | 5.81 |
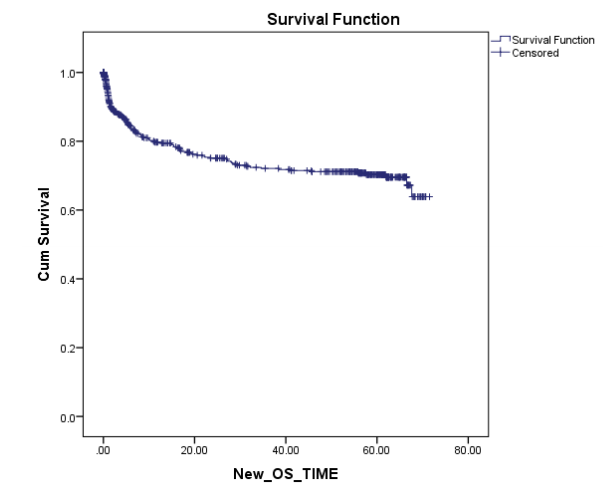
C) Survival:The current Survival rates in PHLG are among the best in the country and are comparable to the rest of the world in most disease subgroups. While OS for all patients registered in 2010 was 69%, we expect this to have further improved for patients starting treatment in subsequent years after the demonstrable impact of our social and supportive care interventions on other parameters, and further refinement of protocols to incorporate risk-stratification. The results of 2010 are even more remarkable when it is weighed with the fact that it most closely reflects outcomes of first-line treatment. This is because from those who unfortunately relapse only a small percentage of our patients are able to undergo salvage treatment which often includes resource and capital intensive options like stem cell transplant, due to severe socio-economic constraints.
Figure: 5-yr overall survival of Pediatric hematological malignancies for all patients registered in 2010 (n=589)
Social Support to Patients: The DMG in collaboration with non-governmental foundations has created a Pediatric Oncology support team constituting a nutrition support team (3 pediatric nutritionists), Infection control & neutropenic care team (medical officer and infection control nurse), social support team (three social workers and 3 volunteers), transfusion support team (a coordinator & volunteer), and emotional support team (4 volunteers), a medical compliance team (1 volunteer), parents guides (2 parents daily by rotation) which is coordinated by Pediatric Cancer foundation called ImPaCCT (Improving Paediatric Cancer Care and Treatment) foundation. This team has been providing various services and has made a significant improvement in the quality of care provided to the patients, has helped decrease the refusal and abandonment rates and has decreased the morbidity and mortality during treatment. The details of support provided as below:
Socio-economic & Accommodation Support: This team has three social workers and 2 volunteers. This team works in tandem with TMH medical social service department, Dorabji Tata trust, Health ministry cancer fund, CANKIDS (an NGO working for welfare of children with cancer), Indian Cancer Society, Nargis Dutt foundation and various individual donors in community. Curable malignancies need to be funded in the initial period to decrease refusal rates through provision of Seed money. All young curable children are adopted by hospital and the rest are supported through various governmental schemes and NGOs. A corpus is being planned to be collected through the “Pediatric cancer Foundation-ImpACCCT” funds. These patients need clean but reasonably priced places to stay in. With the help of St Jude child care centre, Borges home and other organizations, most of our children now stay in beautiful “Home away from home” facilities near TMH and in ACTREC. Poor patients who need to stay in others places are reimbursed for their accommodation if required.
Nutritional Support: - There are 4 dieticians in this team who do the nutritional assessment using as well as dietary counseling and guidance regarding diet especially low cost food supplements. The team has developed LCEF, a low cost enteral supplement along with dietetics department. This team has created many recipes from LCEF including laddoos, theplas, khakras and biscuits. The team has been successfully using these recipes in children with cancer with good response. The team is also running a nutritional support program in which free lunch and a snack are provided to all children since March 2013. More than 25,000 midday meals with snack have been distributed.
Educational Support: The team has, with the help of NGOs called “little-more”, Cankids and Mindsprings have started to provide non-formal education to children taking treatment both in OPDs and wards.
Transfusion Support: Most of our patients come from outside Mumbai and do not have blood or platelet donors. Hence, the team along with Nargis Dutt foundation has started a drive called “Save a Life” to recruit platelet donors from all the colleges & corporate houses in Mumbai and have started a volunteer platelet donor registry at TMH. The target-donors for the initiatives majorly comprised of the college students. This helps provide all our patients blood products whenever required.
Emotional & Treatment Counseling: PHLG has trained volunteers and nurses who guide families regarding the treatment, various supports available and help the families to face the emotional burden of diagnosis of cancer and its treatment.
OPD Timings
General OPD - All Days Except Saturdays
Timing - 09:15-17:30 Hours
Location - Main Building, Ground Floor , Room No.88 .
Priviate OPD - All Days Except Saturdays
Timing - 09:15-17:30 Hours
Location - Main Building, Ground Floor , Room No.88.
| Meetings | Days | Timing |
| Patient Discussion Clinical Joint meeting |
Every 1 st & 3rd Wednesday |
02:30 pm |
| Patient Discussion Clinico-Path Joint meeting |
2 nd & 4th Wednesday |
02:30 pm |
| Administrative Meeting | 1 st Wednesday |
08:30 am |
| Social Support Group Meeting | Every Tuesday | 03:30 pm |
| Surgery days |
Every Monday, Wednesday and Friday |
08: 30 am- 05:30 pm |
| General OPD days |
Monday-Friday Saturday |
09:00 am- 05:30 pm 09:30 am-01:00 pm |
| BMT OPD |
Thurs/Fri (TMH) Mon-Wed (ACTREC) |
10:00 am- 05:00 pm 10:00 am- 05:30 pm |
| Pediatric Social Support GroupGuidance regarding disease and treatment |
Every 4th Wednesday of month |
02:00- 03:30 pm, Choksi Auditorium |
| Pediatric Social Support GroupGuidance regarding nutrition and infection prevention |
Every 2nd Wednesday of month |
02:00- 03:30 pm, Choksi Auditorium |
The DMG has undertaken a total of 68 research studies, 21 were student research (15 completed), 57 studies were initiated by investigators (26 completed) and 5 studies were sponsored clinical trials. The DMG published 10 articles related to its research activity and members also contributed 8 chapters to various books.
|
Total number of clinical trials |
Completed trials |
Ongoing trials |
|||
|---|---|---|---|---|---|
|
Investigator initiated |
Sponsored trials |
Investigator initiated |
Sponsored trials |
Investigator initiated |
Sponsored trials |
|
63 |
5 |
26 |
1 |
37 |
1 (3 studies terminated prematurely) |
Ongoing Research
Original Research Improving Clinical Outcomes:
A) Acute Lymphoblastic leukemia:
Development of indigenous scientifically designed locally feasible protocols: In this regard, PHLG has systematically developed and piloted 3 protocols in collaboration with National Cancer Institute, USA and INCTR, Brussels, Belgium. These include MCP-841, MCP-943 and INCTR-02-04. MCP-841 is the only indigenously developed and highly successful protocol from India and is currently being followed in most pediatric oncology units in the country. Currently, DMG is actively involved in developing the national protocol for treatment of acute lymphoblastic leukemia in India called “ICiCle” (Indian Childhood Collaborative Leukaemia Group) which has
been successfully piloted in 2013. The protocol development meeting was held at Tata Memorial Hospital on December 23, 2012 & March 2014 which laid the foundation of the consensus on backbone of this protocol. This protocol is based on current state of art in ALL and includes risk stratification based on clinical and cytogenetic factors, response to therapy and minimal residual disease which is the first of its kind in whole country. In addition, cranial radiation has been dispensed with from more than 99% patients and high dose methotrexate would be integral part of systemic therapy. This is likely to significantly decrease the treatment related morbidity, cost of therapy and improve the outcome across all risk groups with minimal therapy. Further this would answer 2 key questions related to duration of steroids in induction of ALL as well as choice of anthracycline in delayed intensification. This multicentric protocol involving more than 15 centres across the country is being funded by ICMR and the National Cancer Grid.
Development of Low-cost effective protocol for patient with Socio-economic constraints.
More than 80% of our patients belong to general or poor category, who are unable to afford the current standard therapy. Hence, PHLG has successfully piloted and is currently testing a scientifically designed low-cost ALL protocol since 2005. This protocol was found to be very effective in standard risk ALL with outcomes more than 70% with minimally intensive and inexpensive therapy.
Novel approaches for High risk ALL:-
Many patients with B-Lineage ALL and most patients with T-ALL do not have good outcome with current protocols. Hence, PHLG has been exploring the use of high dose Cytarabine consolidation for improving the outcome of this sub group since 2004. This modified approach has improved the outcome of ALL at our centre to more than 80% in both B & T-lineage ALL, which is comparable to the west.
PHLG is also evaluating the role of the ALL like therapy for biphenotypic leukemia which usually do poorly with AML or Hybrid regimens. Preliminary analysis of these results show that ALL like therapy seems to improve the outcome in this challenging leukemia.
B) Acute Myeloid Leukemia :-
AML:-The group has been using oral metronomic therapy in patients who are ineligible for standard therapy and has been highly successful in achieving remission in children who are otherwise ineligible for standard therapy. The group has also been using oral metronomic maintenance therapy in AML patients with good outcome which are comparable to international outcomes despite higher initial toxic deaths.
APML:- DMG has also studied the role of indigenous metronomic chemotherapy along with ATRA, daunomycin and maintenance chemotherapy in patients with APML. This novel indigenous regimen has shown promising response with limited morbidity and mortality. It is being used in all sick APML patients in our DMG. Also, group published its large experience on characterization of cryptic rearrangements, deletion, complex variants of PML, RARA in acute promyelocytic leukemia recently which is one of the few studies on this aspect from Asia..
C) Lymphoma:
Non Hodgkin’s Lymphoma :-
Treatment:-The group has been evaluating a novel low cost indigenously developed protocol in B-NHL called MCP-842 since 1986. The results of this protocol have been impressive and match the western data. This protocol does not have high dose methotrexate and does not require intensive supportive care unlike the western protocols. In anaplastic large-cell lymphoma, the group has modified MCP-842 with replacement of Vincristine by Vinblastine and addition of maintenance with significant improvement in the outcome. Based on these results at TMH, modified MCP-842 protocol is being used all over the country for B-NHL. DMG has also evaluated the role of Modified BFM-90 (with reduced methotrexate dose) in lymphoblastic lymphoma. The results have been as good as the western protocols. Currently, DMG is piloting immunochemotherapy with rituximab added to MCP-842 for high risk B-NHL.
Prognostic markers: PHLG has prospectively studied the role of early metabolic response in B-NHL and ALCL has found it to be an important prognostic marker which has been presented in SIOP 2013. PHLG would be using early morphologic and metabolic response to delineate high risk group for usingrituximab based MCP-842.
Hodgkin’s Lymphoma: The DMG recently analyzed and presented its experience in treatment and outcome of nodular lymphocyte predominant Hodgkin lymphoma in children in India which is the first large experience on this disease subset from India. The analysis revealed that unlike west, more than 60% children presented with advanced disease. Also, the relapse rates were very high with the ABVD based chemotherapy in this lymphoma but most of the patients could be salvaged with 2nd - line therapies.
In Classical Hodgkin lymphoma, PHLG has found ABVD to be a good regimen in advanced classical HL except children with poor response after 2 cycles on PET-Scan. This data was presented in SIOP 2013 and has been now used to modify the treatment of high risk classical HL with poor early response to dose-dense German protocols. Since 2014, we have been following a stratified approach with a more intensive protocol for all stages apart from I A & II A, while using ABVD in this group.
D) Langerhans Cell Histiocytosis:-
PHLG DMG has prospectively analyzed role of cladribine in the management of relapsed LCH and shown that it is quite effective in salvaging good risk relapse patients.
PHLG has also piloted modified LCH-III with addition of oral metronomic etoposide in high risk multisystem RO+ LCH with promising results. This was presented in SIOP-2014..
E) Chronic Myeloid Leukemia:-
DMG has been studying the biggest cohort of pediatric CML patients; its demographics, response, long term toxicity and survival with Imatinib. It has shown that Imatinib is highly effective and very well tolerated in children. The group has also evaluated the serum level of Imatinib in Indian population and shown that Indian children achieve adequate Imatinib levels with current dosing.
F) Supportive care:- The group has been working on many aspects of supportive care which are as below:-
Nutrition:- The group has been prospectively recording the nutritional status of all the newly diagnosed patients with conventional tools as well as newer tools such as subjective global assessment (SGA) and pediatric subjective global assessment (PSGA). It has shown that novel PSGA is a very good tool for nutritional assessment in children. PHLG has also successfully piloted the use of ready to use therapeutic supplements in children with severe malnutrition with excellent results. This has lead PHLG to initiate a randomized study evaluating the incremental benefit of RUTF in addition to current standard of care in severely malnourished children.
The group with the help of dietetics department has prepared a new low- cost enteral food supplement called LCEF. Many recipes such as Ladoos, theplas, cake, biscuits have been created from LCEF and it is being successfully used in malnourished children with good weight gain. The group is currently planning to prospectively study the nutritional outcome of children with the additional use of appetite stimulants.
Management of Neutropenia and role of growth factors: - The group based on limited data in literature, prospectively conducted a randomized trial comparing low-dose versus standard-dose of growth factors for prophylaxis of neutropenia. The analysis suggests no difference in the end points and this study has the potential to change the practise and significantly reduce the cost of therapy. The group also conducted another randomized trial examining the additive role of standard-dose of growth factors in the treatment of febrile neutropenia along with antibiotics.The analysis suggests that growth factors do not impact the morbidity and mortality in this setting. This is, again, likely to change practice across the world and reduce the unnecessary use of growth factors.
Infections:- The group has studied the predictive markers of sepsis such as CRP prospectively and shown that it is a simple and useful predictive marker. Lastly the DMG has tested a novel indigenous antifungal, liposomal amphotericin-B and shown it to be effective and better tolerated compared to conventional amphotericin-B.
The group recently presented its experience in clinical presentation and outcome of increasing incidence as well as outcome of multidrug resistant bacteria in Pediatric Cancer patients which has limited published data so far. It showed that while MDROs are showing an alarming increase which has increased the morbidity and mortality in children with cancer.
Lastly, PHLG has shown that acyclovir use as secondary prophylaxis in immunocompromised contacts of cases of Varicella infection, is highly efficacious and much cheaper compared to intravenous immunoglobulin.
Projects
- 1 Outcome of Langerhans Cell Histiocytosis (LCH) with a Modified Protocol using Intensified High Risk Induction, and Augmented Prolonged Maintenance- a Single Institution Experience. Project no. 1910; Principal Investigator: Dr (Surg Cdr) Gaurav Narula
- 2 “A Multi-site Prospective Study to Determine Household Out-of-Pocket Expenditure Incurred by Families of Children Newly Diagnosed with Cancer in India” (HOPE Study) Project No. 1736; Principal Investigator: Dr Girish Chinnaswamy
- 3 “A Preclinical Translational Study to Evaluate the Efficacy of scfv-CD28-CD3ζ CAR T-Cells Manufactured from Healthy Volunteers and Patients with Relapsed/ Refractory Acute Lymphoblastic Leukemia in ex-vivo Setting” Project No. 1782; Principal Investigator: Dr (Surg Cdr) Gaurav Narula
- 4 “Predictors and Prognostic value of Early treatment response in Pediatric B-NHL” Project No. 1781 Principal Investigator: Dr Shripad Banavali
- 5 “An observational study to evaluate early treatment response in peripheral blood on Day 15 by flow cytometry in B cell Acute Lymphoblastic Leukemia patients treated with ICiCLe protocol and its correlation with end induction MRD status in bone marrow and outcome” Project no. 1669; Principal Investigator: Dr P G Subramanian
- 6 A prospective randomized study comparing the safety and cost-effectiveness of antibiotic discontinuation without neutrophil recovery (ECIL approach) versus with neutrophil recovery (IDSA strategy) in young febrile-neutropenic patients with cancer Project No. 1639; Principal Investigator: Dr Girish Chinnaswamy
- 7 “A pilot project to determine the expression of adrenergic receptors (ADRs) in the archival formalin-fixed, paraffin embedded (FFPE) tissue blocks of different subtypes of bone or soft tissue Sarcomas- Feasibility study” Project No. 1592; Principal Investigator: Dr. Shripad Banavali
- 8 “A prospective open label randomized active control parellel design comparative pharmacokinetics study of Intramuscular Pegaspargase (Hamsyl) of Gennova Biopharmaceuticals Ltd versus Intramuscular Oncaspar in pediatric patients with relapsed cases of Acute Lymphoblastic Lekemia (ALL)” Project No. 158; Principal Investigator: Dr Shripad Banavali
- 9 A collaborative, multicentre, national trial for newly diagnosed patients with acute lymphoblastic leukaemia (ICiCLe-2014). Project no. 1312; Principal Investigator- Dr B Arora & Dr SD Banavali, CI- Dr (Surg Cdr) Gaurav Narula
- 10 . A Prospective Observational Study of Thrombotic Events Occurring in Pediatric Oncology Patients, and Adolescents and Young Adults with Acute Lymphoblastic Leukemia, and their Causes, Management and Outcomes. Project No. 1493; Principal Investigator: Dr (Surg Cdr) Gaurav Narula, CIs- Dr B Arora, Dr SD Banavali
- 11 A prospective study to investigate the impact of malnutrition on the pharmacokinetics of anticancer drugs in young children. Project no. 1282; Principal Investigator: Dr PA Kurkure. CI-Dr B Arora, Dr (Surg Cdr) Gaurav Narula, Dr SD Banavali
- 12 Clinical Presentation and outcome of Hodgkins disease in children: A retrospective study. Project no. 1107; Principal Investigator: Dr B Arora, CI- Dr SD Banavali
- 13 A prospective observational study to assess the side effects of Imatinib in pediatric patients with chronic myeloid leukemia. Project no. 1278; Principal Investigator: Dr B Arora & Dr SD Banavali, CI- Dr (Surg Cdr) Gaurav Narula
- 14 LCH IV- IVth International study treatment protocol for patients of Langerhans cells Histiocytosis. Project no. 1158; Principal Investigator: Dr (Surg Cdr) G Narula, CI – Dr B Arora & Dr SD Banavali
- 15 Baseline high resolution CT scan thorax for detecting respiratory infection in patients with acute myeloid leukemia at presentation. Project no. 1170; Principal Investigator: Dr SD Banavali, CI- Dr B Arora, Dr (Surg Cdr) Gaurav Narula
- 16 A retrospective analysis of clinical characteristics, treatment and outcome of children with T-cell Non-Hodgkins lymphoma treated at Tata Memorial Centre from 2003-2009. Project no. 961; Principal Investigator: Dr SD Banavali, CI- Dr B Arora
- 17 Retrospective Analysis of Vitamin D Levels in Patients with Malignancies Attending Pediatric Outpatient Department at Tata Memorial Centre. Project no. 966. Principal Investigator: Dr SD Banavali, CI- Dr B Arora
- 18 A prospective randomized, open-labeled parallel group trial of L- asparaginase vs. Prednisolone in a subset of newly diagnosed patients in acute lymphoblastic leukemia presenting with hyperleukocytosis. Project no. 885. Principal Investigator: Dr SD Banavali, CI- Dr B Arora
- 19 Risk Factors, Clinical course and outcome of Chemotherapy induced Febrile Neutropenia in children: a retrospective study. Project no. 953 Principal Investigator: Dr SD Banavali, CI- Dr B Arora
- 20 Validation of BCR-ABL transcript level using ABL as a reference gene by Q- RT-PCR and establishing the conversion factor on an international reporting scale. Project no. 853; Principal Investigator: A Chougle, CI- Dr SD Banavali
- 21 Clinical characteristics and outcome of children with acute myeloid leukemia treated at Tata Memorial Centre over past six years: A Retrospective analysis. Project no. 826 Principal Investigator- Dr SD Banavali, CI- Dr B Arora
- 22 A retrospective analysis of clinical characteristics, treatment and outcome of children with Anaplastic Large Cell Lymphoma treated at Tata Memorial Centre from 1997-2011. Project no. 1312; Principal Investigator- Dr. SD Banavali & B Arora
- 23 Two Vs Four Cycles Of ABVD Chemotherapy And Low Dose Radiation Therapy In Early Stage Favorable Prognosis Hodgkin's Disease: A Randomized Clinical Trial Project No: 110; PI: Dr Maryann Muckaden & Dr S Laskar
- 24 Molecular mechanisms underlying directional migration and retention of B-Cells within stromal microenvironment in Non-Hodgkin’s lymphoma: Role of SDF1/CXCR4 Signaling Axis” Project No: 317; PI – Dr Jyoti Kode
- 25 Burkitt like lymphomas- in search of morphologic, clinical and immune- phenotypic characteristics. Project No: 446; PI – Dr Tanuja Shet
- 26 A phase II single arm prospective study evaluating a novel regimen in the treatment of advanced Hodgkin’s Lymphoma: ABVD-P: addition of prednisolone to ABVD. Project No: 460; PI – Dr Sudeep Gupta
- 27 Comparison of Immune response pattern in nodular lymphocyte predominant Hodgkin lymphoma and T cell/histiocyte rich B cell lymphoma. Project No: 644; PI: Dr Tanuja Shet
- 28 “Minimal residual disease studies in Acute Lymphoblastic Leukemias by flow cytometer. "Project no. 472; PI: PG Subramanian & Dr SD Banavali
- 29 The treatment and characterization of Acute Lymphoblastic Leukemia in Children, Adolescents and Young Adults: INCTR 02 04. Project no. 121; Principal Investigator- Dr SD Banavali
- 30 Comparative Analysis of gene expression profiles of TCR γδ+ VS TCR αβ+ T Acute Lymphoblastic Leukemia. Project no. 268, Principal Investigator- Dr Chiplunkar SV & Dr SD Banavali
- 31 A randomized Comparative trial evaluating the safety and efficacy of Liposomal Amphotericin (Fungisome) versus conventional Amphotericin B in empirical treatment of Febrile Neutropenia. Project no. 257 Principal Investigator-Dr Kurkure PA
- 32 Evaluation of Low cost protocol for treatment of acute lymphoblatic leukemia in Indian children. Project no. 379. Principal Investigators- Dr Kurkure PA & Dr Arora Brijesh
- 33 A randomized open labeled parallel group phase III study of antibiotics alone versus antibiotics plus granulocyte-colony-stimulating factor in pediatric patients with chemotherapy induced febrile neutropenia in a developing country. Project no. 380. Principal Investigator- Dr Arora Brijesh
- 34 To Evaluate the Pharmacokinetics of Imatinib in Indian Patients of Chronic Myeloid Leukemia and the Possible Role of Therapeutic Drug Monitoring. Project no. 622. Principal Investigator- Dr Arora Brijesh
- 35 Study of Nutritional Assessment and Quality of Life in Children Receiving Radiation Therapy. Project no. 531; Principal Investigator- Dr Siddharth Laskar
- 36 Is current dosing recommendation of voriconazole appropriate for Indian pediatric patients? A dose– concentration– efficacy study. Project No 669; Principal InvestigatorDr Gota Vikram, Co-Principal Investigator- Dr Arora Brijesh
- 37A randomized open labeled parallel group phase III study of low-dose versus standard dose granulocyte colony stimulating factor prophylaxis in Pediatric cancer patients receiving chemotherapy. Project No 669; Principal Investigator- Dr Arora Brijesh
- 38To study the expression levels of class I & II Histone Deacetylase (HDAC) genes before and after in-vitro treatment with Valproic acid (VPA) in patients with newly diagnosed, relapsed and refractory acute myeloid leukemia (AML) Project no. 503. Principal Investigator- Chougle A & Dr SD Banavali
- 39IAEA CRP on optimisation of radiotherapy in low resource settings for Pediatric Cancer patients (2008-2012) Project no. 794; Principal Investigator: Dr Sidharth Laskar
- 40Evaluation of stem cells in testicular biopsies of young adult survivors of childhood cancer. Project no 789; Principal Investigator : Dr Purna Kurkure
- 41Role of plasma BNP (Brain, natriuretic peptide) levels as a screening test for anthracycline induced cardiotoxicity: a pilot study. Project no 483; Principal Investigators : Dr Aruna Alahari & Dr Purna Kurkure
- 42“A Phase IIb, Partially-Blinded, Randomized, Active Comparator-Controlled Study to Evaluate the Pharmacokinetics/ Pharmacodynamics, Safety, and Tolerability of Fosaprepitant in Pediatric Patients for the Prevention of Chemotherapy-Induced Nausea and Vomiting (CINV) Associated with Emetogenic Chemotherapy” Project. no. 1238; Principal Investigator: Dr. Shripad Banavali
- 43“A prospective open-labeled randomized control trial of ready-to-use therapeutic food with standard therapy in the management of malignancy-related undernutrition in children” Project No. 1468; Principal Investigator: Dr Maya Prasad
EVENTS ORGANIZED FOR PATIENT EDUCATION & RECREATION
ImPaCCT Foundation School Program




Annual event Hope 2016
Pediatric Birthday celebrations
Moscow Trip 2017




Parent Support Group Meetings
OPD workshops
Visit by the Clown




- For Health Professionals:
- Evidence based management of cancers in India: Guidelines for Acute leukemia: Vol. X (A)2011; Editors- Menon H, Arora B, Sengar M, Nahar A. Publisher Tata Memorial Hospital, Mumbai.
- Evidence based management of cancers in India: Guidelines for Aggressive Non-Hodgkin lymphoma: Vol. IX (B)2010; Editors- Arora B, Sengar M. Publisher Tata Memorial Hospital, Mumbai.
- Evidence based management of cancers in India: Guidelines for Hodgkin lymphoma: Vol. IX (B)2010; Editors- Laskar S, Sengar M. Publisher Tata Memorial Hospital, Mumbai.
- Molecular cytogenetics and Minimal residual disease guidelines for PHLG.
- Febrile Neutropenia Guidelines 2014.
- Tumor Lysis Guidelines 2014.
- Febrile neutropenia guidelines
- For Patients:
- Patient Guidance booklet for general patients
- St. Jude patient Information booklet
- ImpaCCT patient booklet
 Department Office Number: (+9122) 24177000 / Ext.No.A- 7217, 7220
Department Office Number: (+9122) 24177000 / Ext.No.A- 7217, 7220
 Department of Pediatric Hemato Lymphoid,
Department of Pediatric Hemato Lymphoid,
TATA MEMORIAL HOSPITAL, Dr.E.Borges Road, Parel, Mumbai-400-012
Maharashtra, India
 Hospital Fax Number: (+9122) 24101656
Hospital Fax Number: (+9122) 24101656
 Email-id: dmghlp@tmc.gov.in
Email-id: dmghlp@tmc.gov.in
Frequently Visited
Global Navigation
Contact Us
TATA MEMORIAL HOSPITAL
Dr. E Borges Road, Parel, Mumbai - 400 012 India
Phone: +91-22- 24177000, 24177300, 69537300
Fax: +91-22-24146937
E-mail : msoffice@tmc.gov.in(for patient care and queries)/cash@tmc.gov.in(for accounts related)/fundraising@tmc.gov.in (for donors and donation related)/registrar@tmc.gov.in(for education and training)/hrd@tmc.gov.in(for administrative - HRD matters) This email address is being protected from spambots. You need JavaScript enabled to view it.
Copyright © 2016 Tata Memorial Centre. All rights reserved.This website can be best viewed in Microsoft Internet Explorer 9.0+, Chrome, Firefox


Designed and Developed by Mindspace Software Technologies Pvt. Ltd.