 |
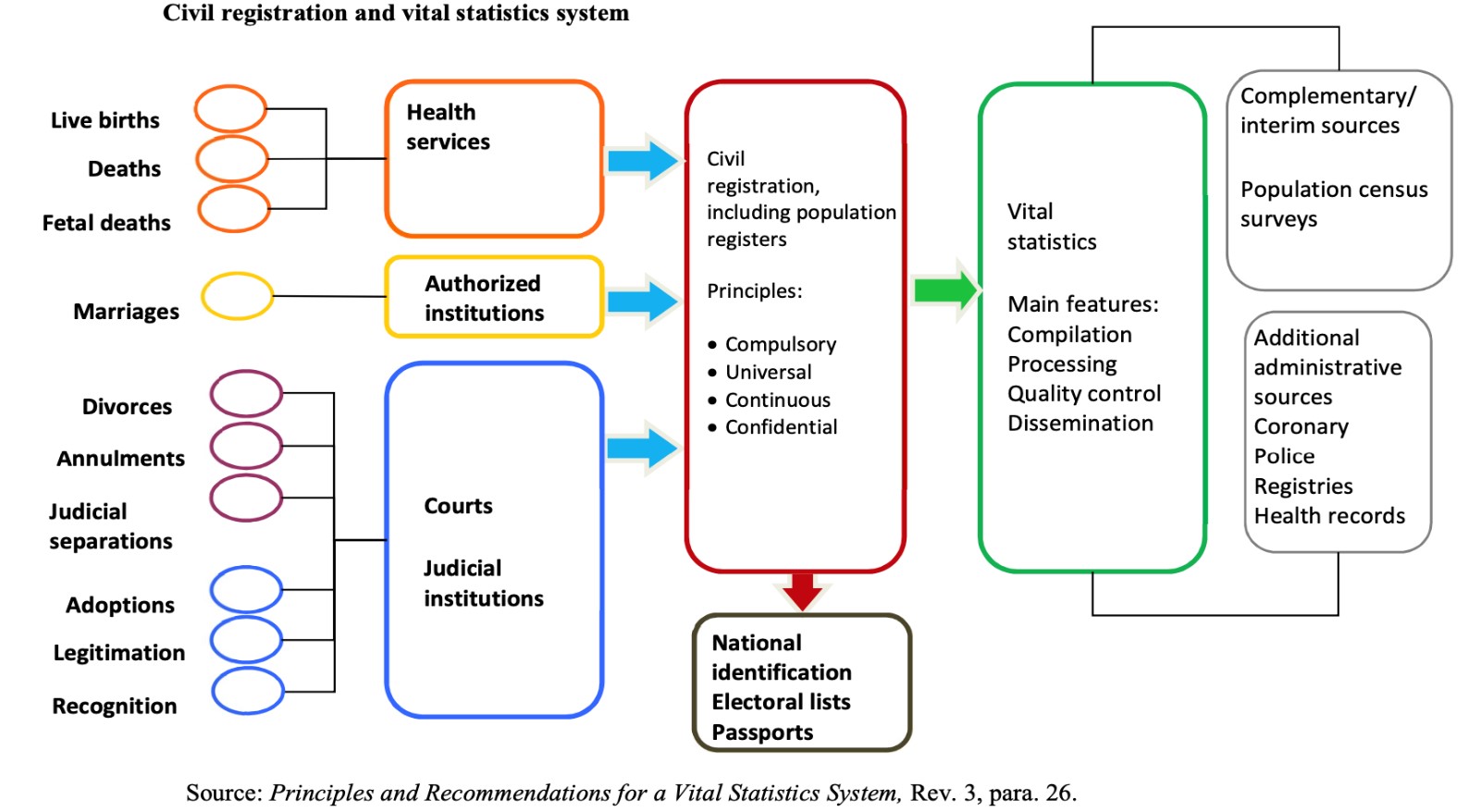
According to the Principles and Recommendations for a Vital Statistics System(2014), ”civil registration is defined as the continuous, permanent, compulsory and universal recording of the occurrence and characteristics of vital events pertaining to the population, as provided through decree or regulation in accordance with the legal requirements in each country (p.65).” In India, the legal basis for the civil registration system is theRegistration of Birth and Death Act, 1969.
Since 1948, the United Nations Statistics Division has had the mandate to develop best practice methodological frameworks and recommendations for civil registration and vital statistics systems. These recommendations include Guidelines on the Legislative Framework for Civil Registration, Vital Statistics and Identity Management(2019) and the Handbook on Civil Registration and Vital Statistics Systems: Management, Operation and Maintenance (2018).
The World Health Organization has the mandate to produce and maintain international classifications on health for governments to use as a common language. One such classification is the International Classification of Disease which is used for morbidity and mortality coding. More information on mortality coding is presented on the coding resources tab.
The vision of this Centre is to support, promote, and advocate for best practices in improving quality of cause of death data.
To achieve this vision, the Centre has established the following goals:
The aim is for the Centre to be a reliable and excellent resource for cause of death activities, including capacity building in mortality coding (ICD-10 and ICD-11), providing support for coding systems establishment, training of doctors in MCCD, and training of analysts and program managers in vital statistics and production of analytical reports.
The Centre will also support advocacy to continue improving standards and efforts in India for high quality cause of death data.
 Dr. Dikshit is an Epidemiologist working in the area of cancer for over three decades, with expertise and experience in cancer epidemiology, molecular epidemiology, surveillance and cancer registries.
Dr. Dikshit is an Epidemiologist working in the area of cancer for over three decades, with expertise and experience in cancer epidemiology, molecular epidemiology, surveillance and cancer registries.
Dr. Dikshit is also the Director of Centre for Cancer Epidemiology at Tata Memorial Centre, Mumbai where he leads multidisciplinary research programs with emphasis on assessing cancer burden, identifying risk factors related to life style and genetics, and studying risk factors for disease progression. He is also engaged in developing education and training programs in Epidemiology to foster research in the country and has served as a faculty for Epidemiology at Tata Memorial Hospital, and other institutions mentoring students in this field.
Dr. Dikshit has been Principal Investigator and co-investigator on numerous nationally and internationally funded epidemiological studies to understand cancers of sites relevant to Indian population such as oral, breast, gall bladder cancers and has trained other researchers who are now leading their own studies.
In addition to research activities, Dr. Dikshit is the regional representative of International Association of Cancer Registries for Asia and Principal Coordinator of IARC Regional Hub for Cancer Registration in Asia, with its headquarters in Tata Memorial Hospital (TMH). With his leadership, Dr. Dikshit has played an important role in expansion of network of population-based cancer registries in India, thereby strengthening cancer surveillance in the country. He has also been actively conducting courses and workshops on cancer registration for registries in India and other Asian countries such as Myanmar, Nepal and Bhutan. The Hub is also responsible for conducting courses on cancer epidemiology, cancer survival and prediction methods.
 Dr. Pankaj Chaturvedi is Head Neck Cancer Surgeon and Deputy Director of Center for Cancer Epidemiology at Tata Memorial Centre, Mumbai. He has been invited as visiting faculty in 44 institutions in 32 countries. He is the editor of the Textbook of Head and Neck Surgery and the Associate Editor of the International Journal of Head and Neck surgery. He has authored more than 200 papers in international peer reviewed journals. He is the Principal Investigator of several pivotal randomized clinical trials. His main area of interest is prevention and early detection of oral cancer. He is the recipient of the prestigious NIH R01 grant for research on tobacco carcinogenesis.
Dr. Pankaj Chaturvedi is Head Neck Cancer Surgeon and Deputy Director of Center for Cancer Epidemiology at Tata Memorial Centre, Mumbai. He has been invited as visiting faculty in 44 institutions in 32 countries. He is the editor of the Textbook of Head and Neck Surgery and the Associate Editor of the International Journal of Head and Neck surgery. He has authored more than 200 papers in international peer reviewed journals. He is the Principal Investigator of several pivotal randomized clinical trials. His main area of interest is prevention and early detection of oral cancer. He is the recipient of the prestigious NIH R01 grant for research on tobacco carcinogenesis.
Dr. Chaturvedi occupies important positions in several prestigious organizations - Secretary General, International Federation of Head Neck Oncologists; Global Coordinator, World Head Neck Cancer Day; Councilor, International Academy of Oral Oncology; Chairman, Oral Cancer Foundation, Indian Dental Association; Founder, Head Neck cooperative oncology group; Founder, Indian Society of Thyroid Surgeons; Founder, Oral Cancer Task Force; Member, International Advisory Board, American Head Neck Societies, Secretary, Action Council Against Tobacco – India. He has received several awards - Excellence in Cancer Care Award, 2017; Nana Palkar Smruti Award for Excellence in Patient Care, 2017; Health Award for Excellence in Oncology, 2016; Iconic Leadership Award, World CSR Day, 2016; Sushruta Award, 2015; BMJ Award for Health Advocacy, 2014; Judy Wilkenfield Award, Campaign for Tobacco Free Kids, 2013; Global Cancer Ambassador, American Cancer Society, 2011; WHO Director General Award for leadership in tobacco control, 2010; Maxwell Robert Byers Award, American Head and Neck society, 2010; Outstanding Young Indian Award, 2008.
Dr. Chaturvedi is the founder of Maharashtra Cancer Warriors that is offering voluntary oncology services in 24 district hospitals of Maharashtra. He is the coordinator of the oncology services on Lifeline Express, world’s first cancer hospital on train. He conceptualized, established and launched India’s first Online Oncology Tutorial that is already being employed by several state governments in India.
Dr. Chaturvedi has tremendous interest in Public Health issues especially related to tobacco, areca nut and alcohol control. He was invited as a speaker to the United Nations Summit on Non-Communicable Diseases, 2011 in New York.
Coordinators and Support Team
Advisory Committee
Partners, Collaborators, and Supporters
To be announced soon!
CRVS Resources
What is Civil Registration?
Civil registration is the system by which a government records the vital events (births, marriages, and deaths) of its citizens and residents. The primary purpose of civil registration is to create a legal document (usually called a certificate) that can be used to establish and protect the rights of individuals. Additionally, civil registration systems serve as a critical data source for the compilation of vital statistics.
The United Nations defines civil registration as "the continuous, permanent, compulsory and universal recording of the occurrence and characteristics of vital events pertaining to the population as provided through decree or regulation in accordance with the legal requirements of a country.”
“Civil registration is carried out primarily for the purpose of establishing the legal documents required by law. These records are also a main source of vital statistics. Complete coverage, accuracy and timeliness of civil registration are essential to ensure the quality of vital statistics."
Vital events that are typically recorded on the register include live birth, death, fetal death, name, change of name, marriage, divorce, annulment of marriage, judicial separation of marriage, adoption, legitimization and recognition. Among the legal documents that are derived from civil registration are birth certificates, death certificates, and marriage certificates.
Please refer to the UN Statistics DivisionPrinciples and Recommendations for a Vital Statistics System(2014) for further information on civil registration systems.
What are Vital Statistics?
Vital statistics is accumulated data gathered on live births, deaths, migration, fetal deaths, marriages and divorces. The most common way of collecting information on these events is through civil registration, an administrative system used by governments to record vital events which occur in their populations.
A vital statistics system is defined by the United Nations "as the total process of (a) collecting information by civil registration or enumeration on the frequency or occurrence of specified and defined vital events, as well as relevant characteristics of the events themselves and the person or persons concerned, and (b) compiling, processing, analyzing, evaluating, presenting, and disseminating these data in statistical form."
Please refer to the UN Statistics DivisionPrinciples and Recommendations for a Vital Statistics System(2014) for further information on vital statistics.
Why do countries register births and deaths?
Birth and death are two important events in the life of any individual as the person's existence starts at the moment of birth and ceases at the moment of death. A person has legal existence between the recorded timings of birth and death. Apart from this legal importance, recording of births and deaths creates vital basic data about a population group. Any planned activity concerning population viz: provisioning of basic necessities like food, housing, health care, education etc can be effective and successful only if this statistical information is correct. Hence, the necessity of registration of births and deaths.
Reference: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4923180/
Overview of Medical Certification of Cause of Death (MCCD)
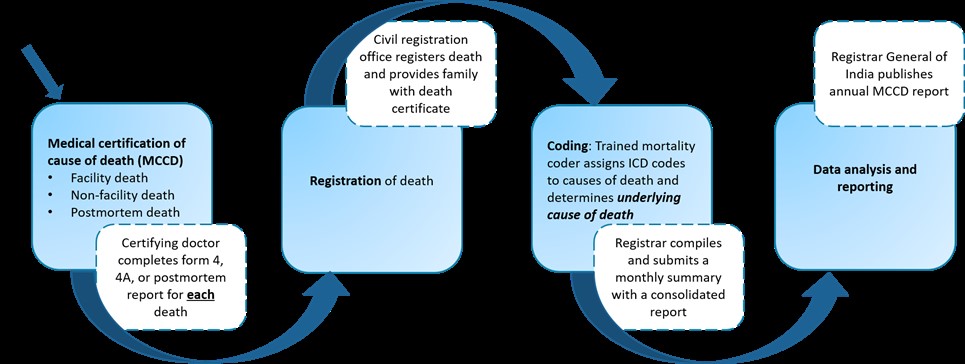
Medical certification of cause of death is the medical doctor’s responsibility for reporting of causes of death using a country’s prescribed form that follows the World Health Organization Standards. (SeeAnnex 7 in ICD10 Volume 2(2019) for more details on the WHO recommended international MCCD form and the reporting recommendations.) Cause of death information is so important for policy and planning that most countries have laws requiring the reporting of cause of death along with each registered death. In theRegistration of Birth and Deaths Act, 1969, medical certification of cause of death reporting requirements are presented in section 10.
Trainings on medical certification of cause of death are available to educate doctors and build their skill and capacity in correctly certifying causes of deaths. In India, the Unit for Strengthening Cause of Death has a free MCCD e-learning course (2021) that bestows 1 CME point via the Omnicuris platform. The Registrar General of India developed a Physicians’ Manual on Medical Certification of Cause of Death (2012) available in hard copy and electronic format. With support from the Data for Health initiative, the University of Melbourne produced an electronic training manual titled, Improving Cause of Death Information: Handbook for doctors on cause of death certification (2016).
For short informational videos on MCCD, please visit the Unit’s Youtube channel which includes a station dedicated to MCCD
MCCD Case Practice (Medical Certification of Cause of Death)
Practice your death certification skills with case examples.
A 67-year-old man presents to the hospital with 6-day history of shortness of breath and dry cough. He was unable to take his temperature at home but feels “hot” and has night sweats. He has a past medical history of hypertension (10 years) and chronic renal insufficiency (6 years). The patient recently traveled from Pune to Mumbai two weeks ago during the lockdown period when Mumbai was experiencing increases in COVID cases and deaths. The travel was necessary to see a specialist for Acute Myelogenous Leukemia (AML) diagnosed only 1 month ago.
The patient was admitted to the hospital with a fever of 103 degrees and transported to the medical unit. Chest x-ray indicated a bilateral pneumonia. He underwent a nasopharyngeal swab for COVID-19. The patient became worse overnight and required intubation. He spent 20 hours on the ventilator prior to death.
The COVID-19 NP swab returned positive.
How would you complete the MCCD form for this case? Think about the causal sequence, time intervals, and underlying cause of death as you consider the correct certification of this death.
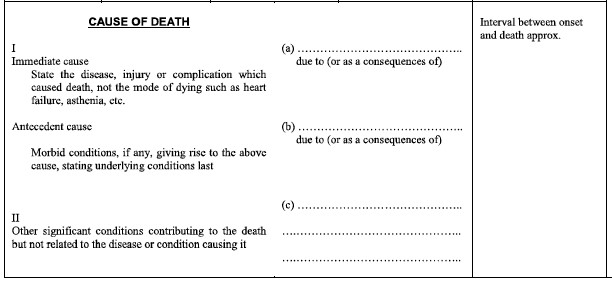
MCCD Case Responses

In this case, the immediate cause of death is ACUTE RESPIRATORY DISTRESS SYNDROME with a time interval onset of 1 day prior to death. ACUTE RESPIRATORY DISTRESS SYNDROME was due to PNEUMONIA with a time interval onset of 1 day prior to death. PNEUMONIA was due to CONFIRMED COVID-19 with a time interval onset of 7 Days prior to death. CONFIRMED COVID-19 is the underlying cause of death in this case and written on the lowest line used- line C.
The decedent has a 10 year history of HYPERTENSION & 1 Month history of ACUTE MYELOGENOUS LEUKAMIA which contributed to the death but was not a part of the causal sequence. Therefore, it is reported in part 2.
Note that the decedent suffered from shortness of breath and dry cough etc. Remember, these are signs and symptoms and you do not write them on the MCCD form. Report only detailed medical conditions in a causal sequence with time intervals on MCCD forms.
The case will be coded with an underlying cause of death of confirmed COVID-19 which in ICD version 10 is U07.1
This case is presented in our COVID coding informational video available on the Unit’s Youtube channel. Watch these short videos for further support on correct death certification and MCCD reporting tips.
MCCCD FAQs
What is Medical Certification of Cause of Death (MCCD)?
For registering a death, identity of the deceased, date and time of death and cause of death are to be provided to the registering authorities. If any of these details are not available, death cannot be registered. Medical certification of cause of death involves establishing the direct cause of death i.e. the disease or condition which the deceased individual was suffering from which led to his or her death, the causal sequence of events that led to the death, as well as the conditions that might have contributed to the death. This involves filling up of the ‘medical certificate of cause of death’ or ‘death certificate’ by a government employed medical officer or a private medical practitioner in the prescribed format.
Reference: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4923180/
Why should medical doctors be the only individuals to medically certify a death?
When confronted with death of a person, the task of diagnosing the occurrence of death and declaring the person dead is the responsibility of the medical officer. Once a death is confirmed, the second task is to decide the cause of death and certify the same. Doctors are qualified to diagnose medical conditions and have an understanding of the pathology of underlying and contributory causes of death, which is essential for certifier of deaths. Hence, forms that are required to medically certify a death have to be filled up by the doctor who has full knowledge of the events which lead to death.
Reference: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4923180/
The international classification of disease, also known as ICD, is a common language used to translate medical terms from a word to a code so that this code. For example, this table below shows how a medical condition written in different languages is the same code in ICD10.
| Medical condition | Language | ICD10 code |
| Obstructed labour due to shoulder dystocia | English | O66.0 |
| Trabajo de parto obstruido debido a distocia de hombros | Spanish | |
| Travail contraint due à une dystocie des épaules | French | |
| प्रसव के समय शििु का कं धा फं सना | Hindi |
Having a universal language with ICD allows for easy comparison of medical conditions across countries. The World Health Organization (WHO) has the mandate to develop and maintain ICD and has developed a range of resources to support countries, including the online ICD10 browser (for the 2019 revisions) and the ICD10 volume 2 instruction manual which presents rules and guidelines for mortality and morbidity coding.
The WHO, headquartered in Switzerland, has relationships with government agencies around the world to serve as Collaborating Centers to support ICD implementation and trainings. The Central Bureau of Health Intelligence within the GOI Ministry of Health and Family Welfare serves as the Collaborating Centre for the WHO Family of International Classification (WHO FIC) in India and South East Asia Region Countries. This Youtube video developed by the Pan American Health Organization (PAHO) and When a coder receives a MCCD form to code, a good practice is to follow these steps to determine the underlying cause of death:
Practice your coding skills with example MCCD form:
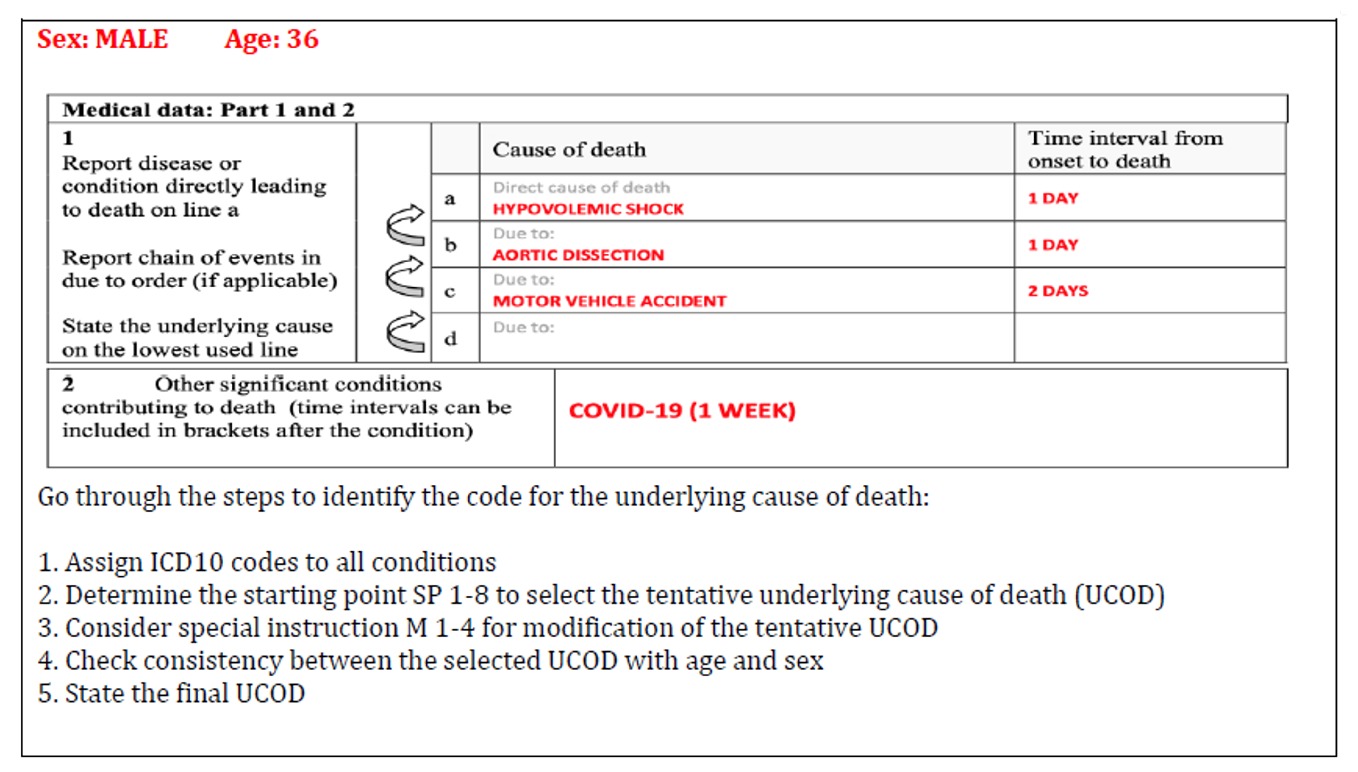
Coding Case 1 Response:
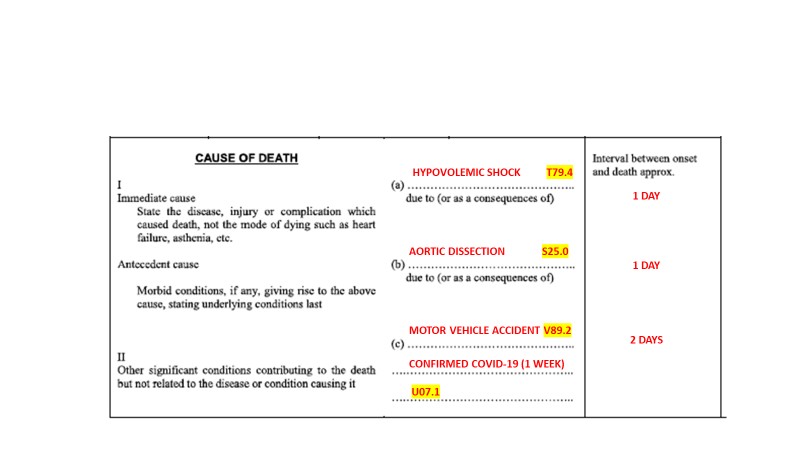
This case was taken from the WHO International Guidelines for Certification and Classification (Coding) of COVID-19 as Cause of Death issued in April 2020.
Coming soon !
Please visit our website for event updates that we will post in this section
USCOD e-Newsletter December 2021 Issue
Glimpses from Virtual Launch of the Unit for Strengthening Cause of Death Data

Discussion with Principal Seretary of Maharashtra at the virtual launch of the Unit
Left to Right : Dr. Nikita Surani, Dr. Rajendra Badwe, Dr. Rajesh Dikshit, Dr. Pradeep Vyas, Dr. Pankaj Chaturvedi

Virtual launch of the Unit |

Dr. Pankaj Chaturvedi speaking at the virtual launch of the Unit |

Planning and coordination for the virtual launch of the Unit |

Launch of the MCCD e-learning course: Demonstration |
Dr. Nikita Surani introducing speakers for |

Dr. Yusuf Hemed presenting at the MCCD webinar during the Unit�s virtual launch |

Speakers of the MCCD webinar discussing a case study with participants |

Dr. Sanchita Sarang�s presentation during the MCCD webinar |

MCCD webinar team |

The team from Unit of Strengthening Cause of Death Data meeting representative from the CDC India office. Left to Right: Mr. Ravindra Pawar, Dr. Pankaj Chaturvedi, Dr. Rajesh Dikshit, Dr. Venkatesh Narayan, Dr. Nikita Surani |

Dr. Rajesh Dikshit speaking at the Second Ministerial Conference on CRVS organized by the United Nations Economic and Social Commission for Asia and the Pacific (UNESCAP) |
 Postal Address:
Postal Address:
Unit for Strengthening Cause of Death Data
Centre for Cancer Epidemiology (CCE)
Advanced Centre for Treatment, Research and Education in Cancer (ACTREC)
Tata Memorial Centre (TMC)
Sector 22, Utsav Chowk-CISF Road
Kharghar, Navi Mumbai
Maharashtra 410210 - India .
Our Email Address
Liaison Officer |
Country Coordinator |
Administrative Officer |
|
Dr Sharvari Mhapankar |
Dr Yagnik Vaza |
Mr Ravindra Pawar |
TATA MEMORIAL HOSPITAL
Dr. E Borges Road, Parel, Mumbai - 400 012 India
Phone: +91-22- 24177000, 24177300, 69537300
Fax: +91-22-24146937
E-mail : This email address is being protected from spambots. You need JavaScript enabled to view it. (for patient care and queries)
This email address is being protected from spambots. You need JavaScript enabled to view it. (for accounts related)
This email address is being protected from spambots. You need JavaScript enabled to view it. (for donors and donation related)
This email address is being protected from spambots. You need JavaScript enabled to view it. (for education and training)
This email address is being protected from spambots. You need JavaScript enabled to view it. (for administrative - HRD matters)
3994137 (1984)